FROM CONCEPT TO PRODUCT
Start your journey today
We're experienced in commercialising new medical devices with clinical creators working in the NHS and beyond - call us today for a confidential, no obligation conversation
10th Sep 2025
SAFIRA®, a pioneering medical device to transform regional Anaesthesia into a single operator procedure is now available to more healthcare institutions across Italy, thanks to a new distribution agreement with Coris Medica.
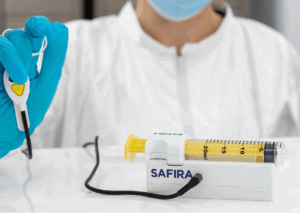
Developed by Medovate in collaboration with UK National Health Service (NHS) anaesthetists, the SAFIRA® system is designed to modify standard regional anaesthesia practice and enhance patient safety. Regional anaesthesia is a widely used clinical procedure to numb a specific part of the body prior to surgery.
Traditionally a two-person technique, regional anaesthesia becomes a single-operator procedure with SAFIRA®, giving anaesthetists full control eliminating the need for an assistant, thereby allowing clinical staff to be redeployed to other essential tasks. The SAFIRA® system also features a unique built-in safety mechanism that automatically halts injection at a calibrated pressure threshold, helping to minimise the risk of nerve damage caused by high-pressured injection.
The system consists of 3 separate components – a proprietary syringe, a driver and an operator. The SAFIRA® operator is available in two variants: a foot pedal operator that sits on the floor during a procedure, and the SAFIRA® Palm Operator – a hand operator that is worn under a surgical glove.
Stuart Thomson, Vice President of Business Development, Medovate Limited
“I am delighted to have reached agreement with Coris Medica who share our mission to make innovative and high-quality medical devices available to healthcare providers and medical professionals. Their proven track record and focus on innovative regional anaesthesia products makes them a natural fit as a distributor partner for our exciting technology developed with NHS clinical innovators. This new partnership means that the clinical, patient safety and cost saving benefits that the SAFIRA® system offers will now be available to regional anaesthetists across Italy. We very much look forward to working with the Coris Medica team.”
Matteo Baffigi, Sales Engineer
We are delighted to affiliate with Medovate to bring this important innovation to healthcare professionals across Italy. Founded in 2003, Coris Medica has more than twenty years of experience in the electromedical sector and a deep understanding of the Italian market. With a nationwide commercial network, we are able to ensure fast and effective access to solutions that enhance patient safety and the quality of care. We strongly believe that SAFIRA® will represent an important paradigm shift in regional anaesthesia, marking a significant step in spreading its benefits and supporting the broader adoption of innovative tools in this field. This partnership marks a significant step in spreading the benefits of SAFIRA® and supporting the broader adoption of innovative tools in regional anaesthesia.
Key benefits of the SAFIRA® device include:
Together Medovate and Coris Medica aim to advance standards in anaesthesia care and support healthcare professional with cutting-edge technology.
We're experienced in commercialising new medical devices with clinical creators working in the NHS and beyond - call us today for a confidential, no obligation conversation