FROM CONCEPT TO PRODUCT
Start your journey today
We're experienced in commercialising new medical devices with clinical creators working in the NHS and beyond - call us today for a confidential, no obligation conversation
4th Oct 2022
Medovate announced today that the game-changing regional anaesthesia technology SAFIRA® (SAFer Injection for Regional Anaesthesia) has been added to the Vizient Group Purchasing Organization (GPO) contract alongside the Konica Minolta SONIMAGE® HS2 Ultrasound System.
Medovate and Konica Minolta Healthcare established a partnership in January 2021 to promote best practices in regional anaesthesia. The SAFIRA® technology was added to Konica Minolta’s UGPro® Solution, which brings together education, procedures and imaging equipment, such as the SONIMAGE HS2, to further expand the use of regional anaesthesia and enhance patient safety.
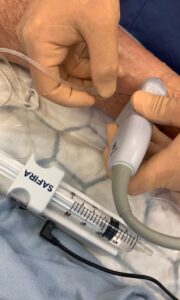 This latest development means that healthcare providers in the USA can now access the SONIMAGE HS2 alongside the additional patient safety benefits provided by the SAFIRA® system.
This latest development means that healthcare providers in the USA can now access the SONIMAGE HS2 alongside the additional patient safety benefits provided by the SAFIRA® system.
Eric Sumner Executive VP, Sales for the Americas from Konica Minolta Healthcare said “The SAFIRA® system is an innovative solution providing additional patient safety benefits during the delivery of regional anaesthesia and complements our SONIMAGE HS2 Ultrasound System”.
Konica Minolta has established ultrasound contracts for its next-generation point-of-care portable ultrasound system SONIMAGE HS2 with USA healthcare company Vizient – the largest GPO in the US, serving over 5,000 not-for-profit health system members.
The current regional anaesthesia procedure is typically for an assistant to inject the anaesthetic solution at the required pressure while the clinician uses ultrasound guidance to place the needle. SAFIRA® is a novel technology, developed alongside specialist clinicians in the National Health Service (NHS) in the UK, designed to increase patient safety during ultrasound-guided regional anaesthesia.
The SAFIRA® system automatically limits injection pressure to a specified threshold to help reduce the risk of nerve injury and improve patient safety. In addition, SAFIRA® also transforms regional anaesthesia into a one-person procedure by enabling a single clinician, an anaesthetist, to conduct the entire nerve block process.
Making regional anaesthesia a single-operator procedure further supports the effective application of resources by enabling the clinician to take full control of the injection process, removing the need for a second operator and freeing up nursing staff for other critical tasks.
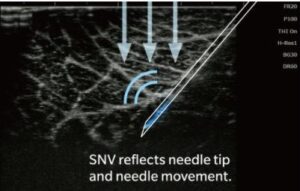 The SONIMAGE HS2 features Simple Needle Visualization (SNV®), an advanced algorithm that improves needle visibility and increases accuracy in needle placement, both for in-plane and out-of-plane approaches. SNV also adjusts the sensitivity of needle visualisation depending on the type of tissue.
The SONIMAGE HS2 features Simple Needle Visualization (SNV®), an advanced algorithm that improves needle visibility and increases accuracy in needle placement, both for in-plane and out-of-plane approaches. SNV also adjusts the sensitivity of needle visualisation depending on the type of tissue.
Stuart Thomson, MD of Medovate said: “We are delighted that through our co-promotional relationship with Konica Minolta Healthcare, our ground-breaking SAFIRA® system is now available alongside the SONIMAGE HS2 through Vizient, the largest GPO in the US. This is a great opportunity for clinicians in the United States to be able to realise the benefits of using these technologies in combination as best practice to improve patient safety during peripheral nerve blocks.”
Both companies are committed to promoting safer regional anaesthesia with solutions that deliver clinical efficiency, simplify use and advance better outcomes for patients.
We're experienced in commercialising new medical devices with clinical creators working in the NHS and beyond - call us today for a confidential, no obligation conversation